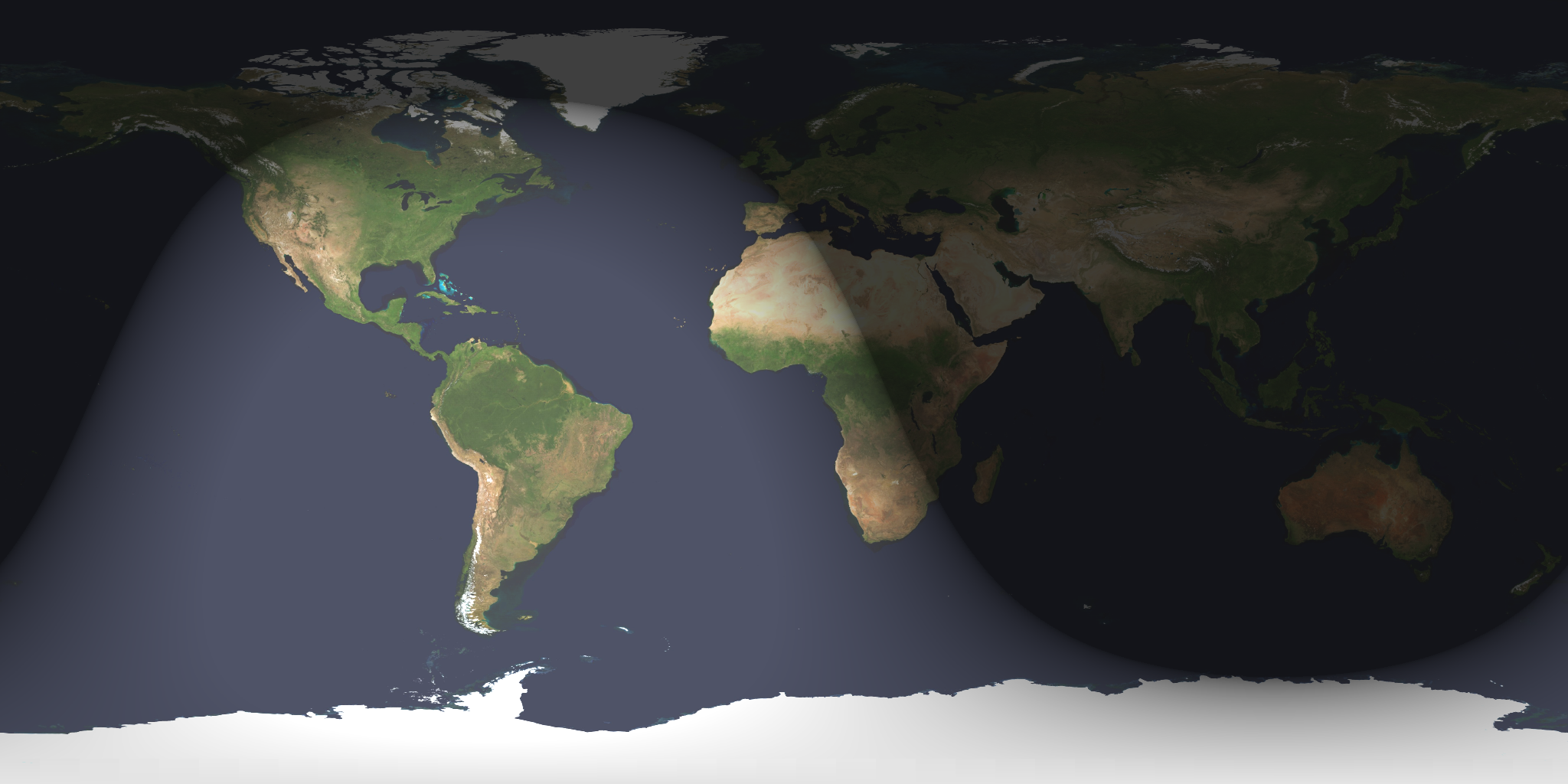
Around We Go
At the time of this post (11:28am EST December 21, 2017), it is officially the Northern Hemisphere winter solstice. Incidentally, it is also my grandmother’s 94th birthday, and since she is one of my loyal readers…Happy Birthday, Grandma!! This means today, the Northern Hemisphere experiences the shortest duration of daylight for the entire year (because of the winter solstice, not my grandmother’s birthday). To be more precise, 11:28am EST is the exact time that the Sun reaches its most southerly point on the path it traces across the Earth (Fig. 1), and begins to move northward. Since the Sun is actually stationary relative to the Earth’s movement, what this really means is that because of the Earth’s tilt on its axis, the solstice (derived from “sun-stopping” or “sun-stationary” in Latin) is the point in the Earth’s revolution around the Sun that it reaches the maximum tilt away from the Sun (Fig. 2), and the Sun appears to stop its southerly motion.

Dealing with Daylight
All living organisms are affected by the reduction in daylight in one way or another. For us humans, this can be anything from the slight annoyance of darkness setting in before we even have a chance to leave work, to psychological effects such as seasonal affective disorder (SAD). While humans are able to cope with the changing light conditions (artificial light, vitamin supplements, etc.) and follow a relatively consistent lifestyle throughout the year, the rest of life on this planet cannot. Most animals compensate by substantially lowering their activity, and in extreme cases hibernate, where they enter a state of reduced metabolic activity and “sleep” until spring. Scarcity of food drives most animals to wait out the dark winter months, and that scarcity begins at the lowest level of the food chain with primary producers. Primary producers rely on the Sun’s energy to turn inorganic compounds (CO2, water) into organic body-building materials, or biomass, as well as compounds used for energy (sugar) through a process known as photosynthesis.
Primary producers have their own ways to deal with the reduction in sunlight. Many land plants and trees will shed their leaves in the autumn and enter a state of reduced growth, conserving vital nutrients until there is enough sunlight to begin growing again. In the ocean, where most primary producers are single-celled organisms (bacteria, algae), the cell numbers will simply be reduced to a just-sustainable level until spring. However, some of these single-celled, photosynthetic algae have a different strategy. These special organisms use the Sun for energy (phototrophy), but may ingest smaller organisms (bacteria, smaller algae) to provide other nutrients for the biological building blocks (heterotrophy). In the low-light, winter months, these “mixotrophs” will begin to rely more on bacteria and diatom (small algae) ingestion to maintain population size, and in some cases, outcompete other microorganisms and thrive.
The Science
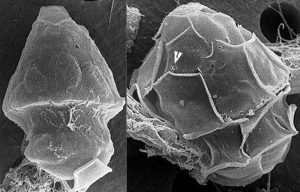
A group of scientists from Maryland and California, led by Dr. Nicole Millette, studied a specific type of single-celled algae called a dinoflagellate that is a mixotroph and common in sunlit oceans around the world. This dinoflagellate (H. rotundata) (Fig. 3) is known to form blooms, or rapidly grow, in the Chesapeake Bay during the winter months. While pressure from algae-eating predators decreases in these months and allows all species of algae to rebound a bit, H. rotundata grows more rapidly than all other members of its community. Dr. Millette’s team postulated that this was due to the algae’s ability to increase its bacteria ingestion when light is low to gain more energy when photosynthesis is reduced. They conducted a combination of environmental observations and laboratory experiments to determine if this was the case.
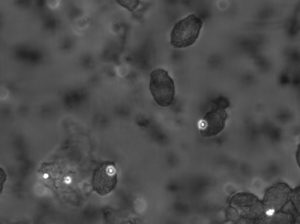
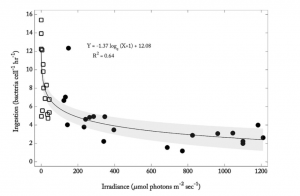
In the first set of experiments, H. rotundata was grown in the laboratory, along with bacteria to be used as prey. The group of scientists exposed the cultures to five different levels of light intensity (irradiance levels) and measured the subsequent rate of bacterial ingestion. Once this was complete, the team then set out to measure bacterial ingestion of H. rotundata in the Choptank River, a tributary of the Chesapeake Bay, in Cambridge, MD. They collected river water into small bottles 20 times over a period of almost two months. Light levels at their sampling location varied by approximately two hours per day over the two-month period. Each day, the researchers added fluorescent microbeads to each collected sample and placed each bottle back into the water to let sit for 30 minutes, allowing the algae time to take up the microbeads as if they were bacteria. At the end of the 30 min, each sample was “fixed” (made biologically inert) to prevent further intake or regurgitation of bacteria by the algae. Back in the lab, Dr. Millette was then able to count the fluorescent microbeads inside each one of the algal cells and correlate that number to the irradiance on the day the sample was taken (Fig 4).
Beating the Odds
The scientists found that H. rotundata does, in fact, increase its bacterial consumption as light levels go down (Fig. 5). In both the laboratory experiments and field work, the algae appear to shift their already unique survival strategy towards obtaining more of their energy from ingested organisms during the long winter months. This study is important and noteworthy in that it expands our knowledge of how organisms cope with variations in their environment, and in some cases, thrive when other members of a community may struggle under the same conditions.
I am completing my doctorate at the Graduate School of Oceanography at the University of Rhode Island where I study the community structure and evolution of deep-sea sediment bacteria. I have also been an adjunct professor at the Community College of Rhode Island for two years. I earned a B.S. in Aerospace Engineering from the University of Miami and spent 12 years in the US Navy driving submarines before coming back to grad school.