
Hernández-Orts JS, Brandão M, Georgieva S, Raga JA, Crespo EA, Luque JL, et al. (2017) From mammals back to birds: Host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin, Spheniscus magellanicus. PLoS ONE 12(10): e0183809. https://doi.org/10.1371/journal.pone.0183809
Background
Being a parasitic organism is hardly a piece of cake. Not only does your lifecycle often involve multiple hosts, but when you do reach your final destination, you have to make sure not to draw too many resources from your home. After all, a parasite with a dead host isn’t doing itself any favors. So, throughout evolution, parasites have evolved to be resilient creatures equipped to deal with specific host organisms.
Specificity has its perks, but room for host flexibility is always a good backup plan. Typically, this flexibility is seen in parasites being capable of infecting species closely related to their original host—like flatworms that can infect wolves, foxes, and domestic dogs. Seems like an easy enough leap. But every one in a while something known as a “host-switch” occurs, where parasites find they can put down roots inside distantly related animals. This may have happened along the coast of South America where adult acanthocephalan (“thorny-headed worm”) specimens (Fig. 1) have been found in the digestive tracts of both pinnipeds (the South American fur seal and sea lion) and the Magellanic penguin.
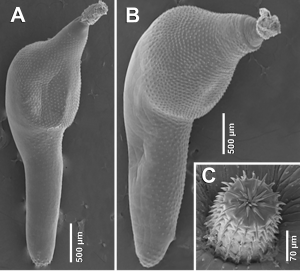
So how has this parasite pulled off the switch from pinniped to bird? Jesús Hernández-Orts and his team set off to find out.
Methods
Newly, naturally deceased specimens of Magellanic penguins, South American fur seals and South American sea lions were collected from beaches in Brazil and Argentina, dissected, and surveyed for parasites. All parasites were preserved for analysis, aged, and sexed; adult females were processed even further to estimate how fertile they were. Physical characteristics were noted to see if host species caused differences in parasite growth/size. Finally, and perhaps the most satisfying of all, came DNA extraction where the full genome of these thorny-headed worms was sequenced before scientists focused on two individual genetic sections.
Bigger Picture
As with most things in science, the results were hardly clear-cut. However, they did reveal the interesting evolutionary history of this specific thorny-headed worm. Based on the genetic analysis, Hernández-Orts and his team reconstructed a potential family tree that indicates the acanthocephalan found in Magellanic penguins may have had an ancestor that had more success using marine birds as hosts. It’s possible the modern parasite is using those genes to better infiltrate the penguin system.
However, just because the parasite is capable of surviving in the penguin’s gut, does not mean it is thriving. There were three distinct trends in the specimens collected. First, infection rates in the penguins were much lower than those in the pinnipeds. Second, the sex ratio of females to males in the penguins was heavily skewed towards females. And third? There were more non-gravid (non-fertile) females than fertile females in the penguins when compared to those in pinnipeds.
A possible explanation for the infection rate could lie in decreased contact with the parasites over years. Magellanic penguins feed on animals not typically known for carrying acanthocephalans—but if yearly diet shifts driven by prey availability forced the penguins to eat more of a heavily-parasitized species, it could result in differing infection rates. As to the skewed sex ratio, female acanthocephalans typically live longer, but the relative absence of male parasites suggests either a lower infection rate by males or a higher mortality after infection. Finally, the fact that there were more non-fertile females in the penguins, hints that although the birds are providing a home, it’s less than comfortable for the parasites as they are not reaching a reproductive phase.
Parasitic relationships are complex and nuanced—there are so many things we still don’t know. But this “host-switch” sheds light on an interesting question: what if flexibility in hosts isn’t just about staying within a family of animals, but rather keeping to animals that fill similar ecological roles? At this point, the Magellanic penguins don’t seem in any danger of toting around hundreds of these unwelcome guests, and the worms obviously still have some evolving to do before they’re fully equipped to survive and reproduce inside the penguins…but having those dusty old genes in their genome is certainly giving them a leg (or hook) up.
I am a former PhD student from the University of Rhode Island, having discovered my love of teaching and informal science education in part through OceanBites! Since departing academia, I’ve focused on creating educational content for visitors at the New England Aquarium, Chincoteague Bay Field Station, and now the National Aquarium. I’ve also dabbled in co-creating a comedy/brainstorming podcast, ThunkTink, and enjoy getting lost in nature with my dogs.


