Citation: Song, M., Schubotz, F., Kellermann, M. Y., Hansen, C. T., Bach, W., Teske, A. P., & Hinrichs, K. (2021). Formation of ethane and propane via abiotic reductive conversion of acetic acid in hydrothermal sediments. Proceedings of the National Academy of Sciences, 118(47).https://doi.org/10.1073/pnas.2005219118

Hydrocarbons – molecules made up of only hydrogen and carbon atoms – are the key components of oil and natural gas, which currently power our energy grid. However, the supply of oil and natural gas is not unlimited. There is a finite amount in the ground, and while the supply hasn’t come close to running out, energy companies have begun to rely on more complicated, expensive methods to extract it (i.e. offshore drilling and fracking).
The main reason oil and natural gas supply is limited (and the reason they’re referred to as “fossil” fuels) is that hydrocarbons typically form slowly – under intense heat and pressure. Organic material in the ocean (the remains of dead organisms) sinks to the seafloor and is subsequently buried. Over millions of years, that organic material is buried deeper and deeper into the Earth, where it’s heated and pressurized. At specific depths known as the “oil window” and “gas window”, the heat and pressure are great enough to transform buried organic material into oil and natural gas, respectively.
This process is called thermogenic hydrocarbon formation (“thermo” = heat, “genesis” = origin).
Isotopic Signatures
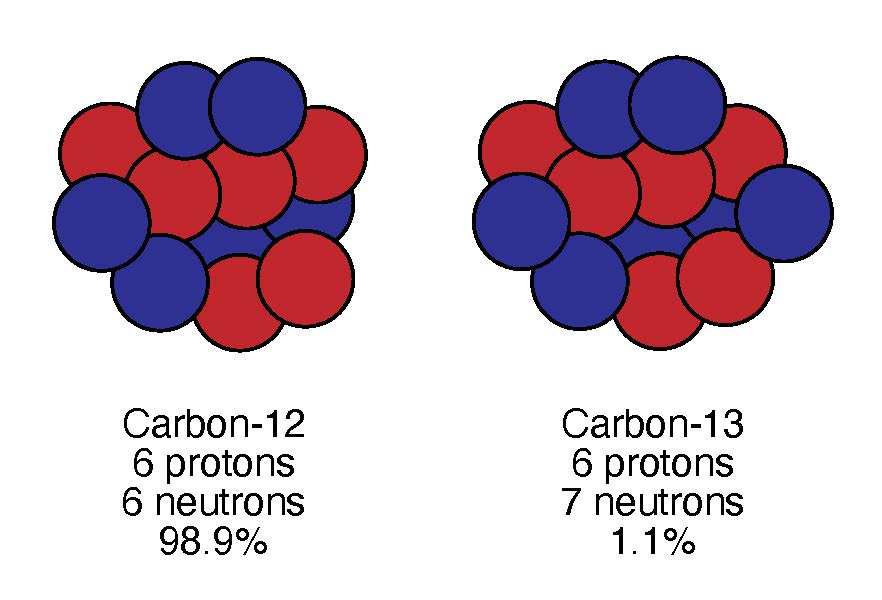
As a result of the thermogenic formation pathway, the hydrocarbons in oil and natural gas have a particular isotopic signature. Isotopes are two atoms with the same number of protons, but different numbers of neutrons. Carbon-12 contains 6 protons and 6 neutrons, for example, while carbon-13 contains 6 protons and 7 neutrons. An isotopic signature is the ratio of different isotopes (in this case, carbon-13 to carbon-12) in a particular material. The C13:C12 isotope signature is commonly used to determine how hydrocarbons in different environments are formed.
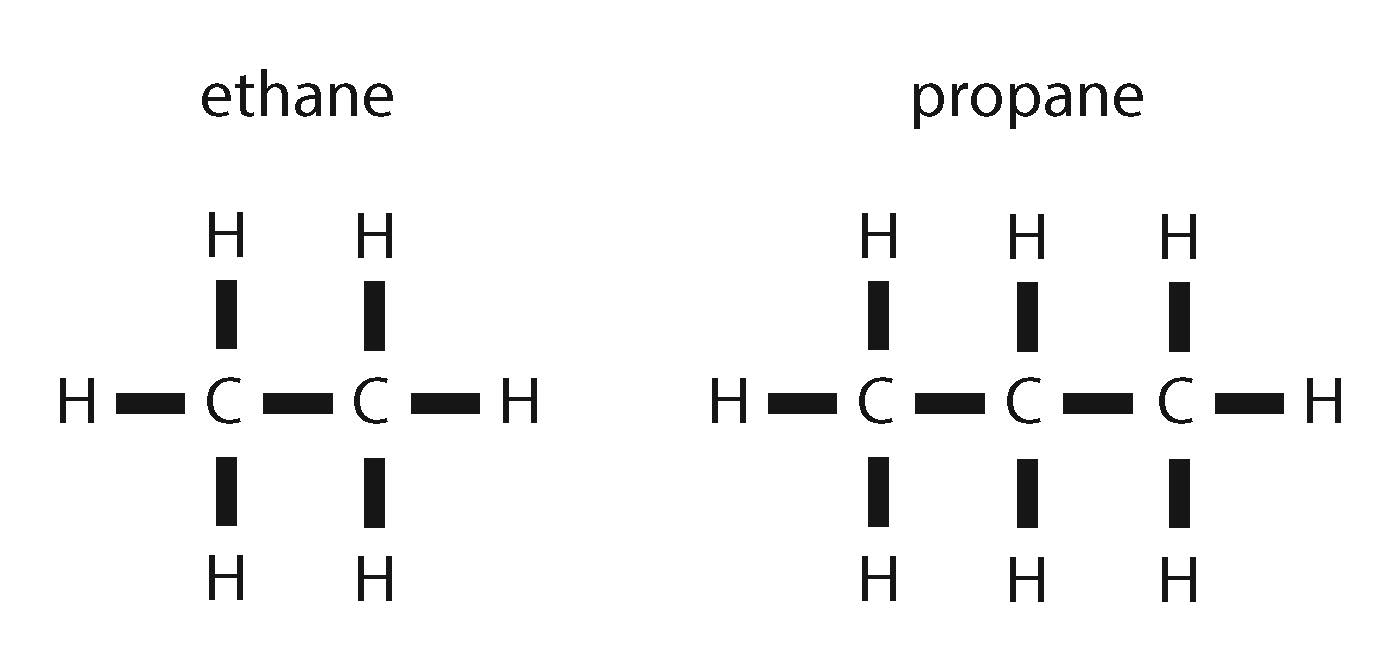
Hydrocarbons formed through the thermogenic pathway result from the breakdown of larger molecules. Hence, the ratio of carbon-13 to carbon-12 decreases as the size of the hydrocarbon decreases. The science behind this is complicated, but in short, it’s easier to break C12 off a larger molecule than C13 because it weighs slightly less. The smaller the hydrocarbon, the more breaks it has experienced from larger molecules, and the less likely it is to contain C13. For example, thermogenic ethane, which is a 2-carbon-long chain, has a lower C13:C12 ratio than thermogenic propane, which is a 3-carbon-long chain.
Large to Small? Or Small to Large?
However, a team of scientists from the Center for Marine Environmental Sciences in Bremen, Germany made an unusual observation. In hydrocarbons sampled from the Guaymas Basin in the Gulf of California, the team noticed C13:C12 ratios that instead increased as the size of the hydrocarbon decreased. This indicated to them that the hydrocarbons were not a product of thermogenic processes. Furthermore, they could not have been a product of biogenic processes (hydrocarbon formation by specialized microorganisms), as the temperature at the sampling sites exceeded what the microorganisms can usually tolerate.
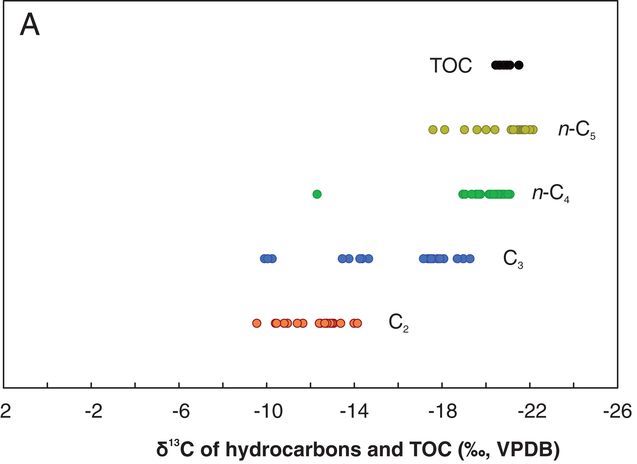
After a bit of brainstorming, the research team deduced that another process could be happening in Guaymas Basin. Temperatures there were high enough at the ocean floor to form oil and gas extremely rapidly – without much burial. The team also observed that the concentration of small, carbon-containing volatile fatty acids (VFAs) was unusually high. Perhaps hydrocarbons were being built up from these small VFAs, rather than resulting from the breakdown of a large molecule?
They team tested their theory in the laboratory, making sure to mimic the temperature and pressure conditions of Guaymas Basin. Indeed, they observed the production of hydrocarbons with the unusual isotopic signature.
Implications
Rather than resulting from the breakdown of large carbon compounds, the Guaymas Basin hydrocarbons instead result from the fusion and substitution of small carbon compounds. This new hydrocarbon formation pathway – and its diagnostic isotope ratio – can now be sought in other similar environments across the ocean floor.
Furthermore, the scientific team warns that the discovery of this process means that other researchers should consider its effects on the isotopic ratios of other hydrocarbons. Isotopic signatures have traditionally been reliable fingerprints of specific formation processes. However, the new pathway, which can occur in conjunction with others, is considerable enough to affect and confound isotope ratios in hydrothermal environments like the Guaymas Basin.
Simply put, isotopic signatures may tell a slightly muddled story about how hydrocarbons are formed. In proposing an entirely new pathway, this study is an important reminder that the ocean still holds plenty of secrets.
I’m a PhD candidate in Earth System Science at Stanford University, and I study how microbes in deep ocean sediments produce and consume greenhouse gases. I’m a native of the landlocked state of Minnesota, so I’ve always been fascinated by the ocean. When I’m not in the lab, I love to race triathlons, forward “The Onion” articles to friends and family, and hike with my hound dog Banjo.


I’m sure you realize the implications since this indicates what I have suspected for many years that most hydrocarbons have been and still are being created using the energy of the core of the Earth.