Reviewing: Savio, S., Farrotti, S., Di Giulio, A., De Santis, S., Ellwood, N. T. W., Ceschin, S., & Congestri, R. (2022). Functionalization of Frustules of the Diatom Staurosirella pinnata for Nickel (Ni) Adsorption From Contaminated Aqueous Solutions. Front. Mar. Sci, 9, 889832.
For many of us, clean drinking water is often taken for granted. We can easily turn on a tap or purchase a bottle of water at a store. But according to the World Health Organization, one in every 10 people on the planet does not have access to safe drinking water, and one child under 5 dies every 20 seconds from diseases caused by unclean water. One major pollutant of safe drinking water is heavy metals. Metals such as mercury, lead, arsenic and nickel are relatively denser and heavier than most metals, so they are somewhat loosely defined as “heavy” metals. But while these metals are found naturally on soil, water and air, they can also accumulate in our body over time and threaten our health. In particular, nickel is a very widely used metal in our everyday lives – in pots and pans, vehicles, smartphones and laptops – and it can get discharged in industrial wastewater into the environment. Removing heavy metals such as nickel from contaminated waters is important for public health because exposure to high amounts of nickel can cause skin allergies, heart and kidney diseases, lung fibrosis and cancers.

Currently materials such as activated carbon (in your standard Brita pitcher filter) and clay minerals are most commonly used to treat heavy metal contaminated waters. Because contaminated water is such a huge public health threat, scientists and engineers are continuously working on finding alternative materials that can remove metals more effectively and can be produced sustainably and cheaply. One team of scientists is focusing on a potentially effective and environment-friendly alternative: diatom frustules.
Okay, what is a diatom frustule?
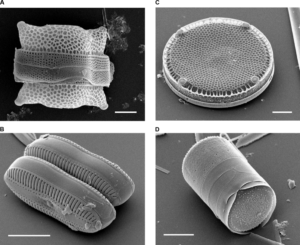
Diatoms are algae that live in the oceans. These can be found almost anywhere in the ocean very easily. Like other algae, diatoms photosynthesize, taking up carbon dioxide from the atmosphere to produce oxygen and sugar. In fact, about one fifth of the oxygen you’re breathing comes from diatoms. There are companies that grow diatoms in the lab, to extract pigments which can be used in pharmaceutical and cosmetic industries, and to extract oils that can be used as biodiesels. But this team of scientists wanted to utilize a different part of diatoms – their “frustules”.
Frustules are hard layers made of silica on the outside of the cell which act as an exo-skeleton. Because diatoms take up nutrients and release waste through their frustules, they design the layers in very complex shapes to maximize surface area. For diatoms, this means that the frustules have more areas to take up food, but for these scientists, this meant that the frustules have more areas where heavy metals can stick to. The unique design of frustules can filter out heavy metals when they are added to contaminated water!
The scientists also took an extra step of putting a layer of coating over the frustule surfaces, so that metals can be even more efficiently removed from contaminated water. This coating works like a special glue just for nickel, so while the coated diatoms are not actually sticky, nickel will get stuck to the coatings much faster than it would on the surfaces of frustules without the coatings. To test how efficiently the coated diatom frustules could remove nickel compared to the uncoated frustules, the scientists added both types of frustules into water contaminated with nickel and left them for a day. While the raw frustules without coating removed only about 40% of nickel over a day, the coated frustules removed over 95% of nickel in just 10 minutes! The coated frustules turned into a dark red color from white, which clearly showed that nickel in water has reacted with the coated frustules and was removed from water.

The scientists plan to do additional tests to see whether the coated diatom frustules can remove other heavy metals as efficiently as nickel, and they plan to find more efficient ways to improve the coating at the frustule surface to further reduce the costs associated with coating. But this is already a very promising result, as these coated frustules are still much cheaper to produce in large amounts than other materials can remove nickel from contaminated water as efficiently. Who knows, maybe we will be using Brita filters with coated diatom frustules in the future!
I am a PhD student in chemical oceanography at University of Washington. I am studying how different forms of metals in the ocean are shaping microbial communities in the North Pacific Ocean. When not working, I like going for a walk, visiting farmers’ markets and playing keyboard.

