Basic Needs
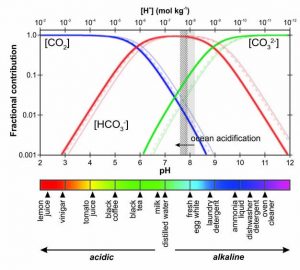
If you are familiar with some of the discussions concerning climate change, then you have probably heard of ocean acidification. Contrary to how this may sound, our oceans are not actually turning into boiling cauldrons of acid, which is probably a disappointment to all of my James Bond-villain readers out there. Seawater is comfortably situated towards the basic side of the pH scale, and there really is no danger of moving to the acidic side. Ocean acidification just means that due to the input of CO2 (carbon dioxide) into the oceans from the atmosphere, the pH is shifting towards the acidic side, and in fact becoming more neutral (Fig. 1). As humans, we may all enjoy a nice, neutral glass of water, but this has detrimental effects for many of the organisms that live in seawater due to the complicated balance of chemicals on which these organisms rely.
Carbon Comfort
One of the most complicated and delicate chemical balances in the ocean revolves around the way CO2 is dissolved. Probably the most familiar way CO2 is dissolved in water is in its gaseous form. Most of us have had a carbonated beverage at one point in our lives, and we know that the drink will continue to create bubbles until it becomes “flat”. This is because the CO2 molecules are distributed evenly in the solution and only bubble when enough of them get together to form a gas. In seawater, things work a little differently. While there is still some dissolved CO2, most of the carbon dioxide absorbed from the atmosphere goes on to add another oxygen atom and become carbonate (CO32-). Carbonate is the shell-building material used by organisms ranging from microscopic algae to giant marine mollusks. They combine the CO32- with calcium (Ca2+) to create calcium carbonate (CaCO3) which is the same material found in chalk and stomach antacid tablets, and is the primary component of seashells and white-sand beaches the world over. However, there is one more key component in this system. CO32- also combines with hydrogen atoms to form bicarbonate (HCO3–), which then makes the carbonate unavailable for shell-building. The reason the pH of the ocean is so important is because the percentages of each of these carbon-based compounds is dependent on pH. At the normal ocean pH (about 8.2), there is plenty of CO32- for organisms to combine with Ca to build shells. But, as the that number lowers, the amount of CO32- goes down, and the other two compounds (HCO3– and CO2) go up (Fig. 1), making shell-building more difficult and energy-intensive.
Ptera-Firma
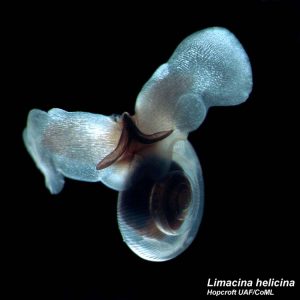
As ocean acidification has been investigated, probably the most widely sighted organism used to illustrate the harm caused to shell-building sea creatures is a tiny, shelled mollusk called a pteropod (Fig. 2). These are small, swimming sea snails that make their delicate shells out of CaCO3. Scientists have noticed that as available CO32- declines with dropping pH, the shells of these creatures are becoming thin and brittle. It is a very immediate, and visible effect of atmospheric CO2 increase. If we have already begun to see such significant changes in the most delicate of ocean organisms, then the destruction of the larger, more robust animals will inevitably follow as we proceed on our current trend of fossil fuel burning. While not a solution to the problem of ocean acidification, one group of scientists noticed something interesting about a mechanism used by the pteropods to repair damage to their shells caused by failed predation attempts and other damage. In areas of the shell where there is damage, the pteropod will secrete CaCO3 to the inside of the shell to perform a patch, and these scientists thought that same mechanism may be used to strengthen the shells that become thin and brittle due to ocean acidification.
The Science
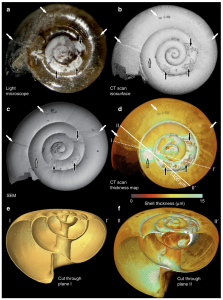
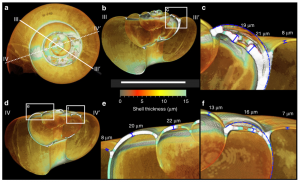
This team of researchers from the US and UK, led by Dr. Victoria Peck, set out to determine if the identified repair mechanism could be used to mitigate the effects of ocean acidification. They collected water samples near the Arctic Archipelago of Svalbard during a research cruise in the summer of 2012. Specifically, they filtered these samples to collect the tiny pteropod, Limacina helicina (Fig. 2). Once in the lab, the scientists employed three different types of imaging techniques to look for possible shell damage, and subsequent repair. They used light microscopy to identify areas of possible damage based on shell transparency, and a scanning electron microscope (SEM) to identify specific deformities in the shell surface. Once the team had created a sub-set of shells that had clear evidence of external damage, they used a computed tomography (CT) scan to create cross sections of the shells to measure shell thickness (Fig. 3). These scans then allowed the researchers to measure the exact shell thickness at damaged sites, as well as determine locations where external shell loss was compensated for by internal repair.
Implications
Based on the images collected, Dr. Peck and her team conclude that L. helicina are able to conduct self-repair when their shells are damaged, and are able to thicken their shell walls from the inside when the surrounding waters do not support the CO32- necessary for healthy shells (Fig. 4). However, this is not a solution to the problem of ocean acidification. The metabolic cost to L. helicina for conducting such repairs is great, and in polar waters where food is often limited, this mechanism will not overcome the damage due to climate change. Actions to drastically reduce fossil fuel emissions are still greatly needed, and this study may even help to support the fact that while many animals are resilient to environmental changes, there is only so much that can be accomplished against such harsh, and drastic changes to their habitat.
I am completing my doctorate at the Graduate School of Oceanography at the University of Rhode Island where I study the community structure and evolution of deep-sea sediment bacteria. I have also been an adjunct professor at the Community College of Rhode Island for two years. I earned a B.S. in Aerospace Engineering from the University of Miami and spent 12 years in the US Navy driving submarines before coming back to grad school.