Citation: Shiozaki, T., Fujiwara, A., Inomura, K., Hirose, Y., Hashihama, F., & Harada, N. (2020). Biological nitrogen fixation detected under Antarctic sea ice. Nature Geoscience, 13, 729-732. https://doi.org/10.1038/s41561-020-00651-7
[hr]
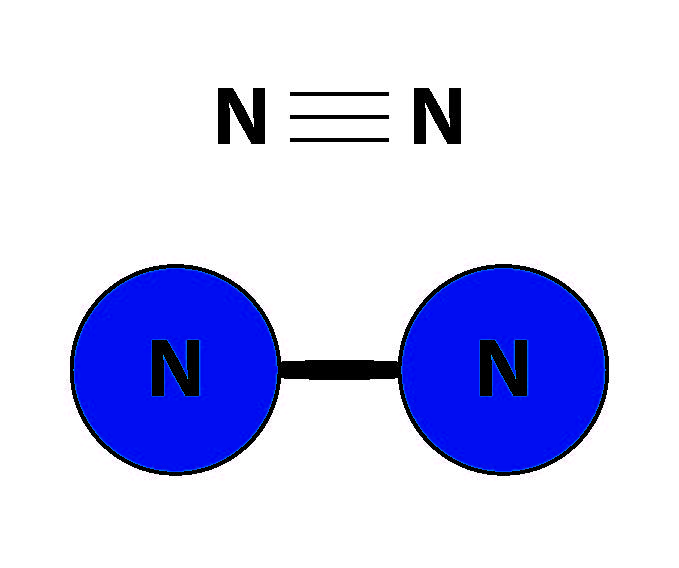
The element nitrogen is a component of DNA, proteins, and other biomolecules, making it indispensable for life on earth. Organisms can get their nitrogen from a wide variety of compounds; nitrate (NO3–), nitrite (NO2–), and ammonium (NH4+), for example, can all be used by a vast number of macro- and micro-organisms. But the most abundant form of nitrogen – nitrogen gas (N2) – cannot be used, despite making up 71% of the air we breathe. Unlike other nitrogen compounds, the N2 molecule contains a triple bond, which holds its two nitrogen atoms together in a vise-like grip (Figure 1). This triple bond is incredibly hard to break, and only a special class of organism, called a nitrogen fixer, is able to convert N2 into forms of nitrogen that are more “bioavailable” to other living things.
Nitrogenase: Benefit or Burden?
Nitrogen fixers utilize a special enzyme called nitrogenase to pull apart the N2 triple bond. Nitrogenase is a convenient enzyme to have, because it means an organism has a virtually unlimited source of nitrogen. They can literally pull it out of the air around them. However, nitrogenase is a large, energetically-expensive enzyme to make and carry around. Having it would be a bit like hauling your garden around in a wheelbarrow forever. You might never run out of vegetables, but the extra load will restrict how fast you move, and how much energy you have for other activities.
To make nitrogenase, organisms need to have specific genes in their cells that instruct them how to make it. The most well-known of these genes is called nifH. To summarize, if an organism doesn’t have the nifH gene, it cannot make nitrogenase, and therefore cannot fix nitrogen.
Marine microbiologists typically find more nifH genes in parts of the ocean where bioavailable nitrogen (i.e. nitrogen in compounds other than nitrogen gas) is not abundant. In these places, nitrogen fixers should have a competitive advantage over other organisms, because they can use nitrogen gas as a nitrogen source. In other places – where bioavailable nitrogen is abundant – nitrogen fixers get very little benefit from nitrogenase, and their extra baggage should put them at a disadvantage.
An Unlikely Home for Nitrogen-Fixers
The Southern Ocean, which encompasses Antarctica, is a perfect example of the latter. It is comparatively rich in bioavailable nitrogen compared to other parts of the ocean, and as a result, it was long-thought to be lacking in nitrogen fixers. A second factor is also working against nitrogen fixers here: low iron concentrations in seawater. The enzyme nitrogenase contains a lot of iron (just like the iron-rich protein hemoglobin in your blood), so nitrogen fixers need a lot of it. However, the Southern Ocean is far from any iron sources, like continents and wind-blown dust, so there’s very little iron around.
Based on the combination of these two factors – the abundance of bioavailable nitrogen and the lack of iron – scientists assumed that nitrogen fixers would not be abundant in the Southern Ocean. However, a Japanese research team decided to verify this theory. They took a research vessel to the Antarctic and collected samples of ocean water from 21 sites – some close to the coast, and some in open water. They brought the water samples back to the lab, extracted the DNA inside, and used a technique called PCR to search for the nifH gene. From this, the team made an unexpected discovery: there are indeed organisms in the Southern Ocean that can transform N2 into bioavailable nitrogen, and they are most abundant near the Antarctic coast.
But why was our original assumption so wrong?

Sea Ice Provides a Clue
The researchers speculate that the sea ice in Antarctica (Figure 2), which contains trace amounts of iron scraped away from the Antarctic continent, is providing a source of iron for the nitrogenase enzyme. As the ocean around Antarctica warms up, the sea ice melts, releasing iron into seawater and contributing to the growth of nitrogen-fixing organisms.
However, it’s still not fully understood why Southern Ocean nitrogen fixers aren’t disadvantaged by the abundance of bioavailable nitrogen. One reason could be the organism responsible. The most abundant nitrogen-fixing organism across the 21 sites was a bacterium called UCYN-A. This bacterium is found all across the globe, and across a wide range of seawater temperatures. Just as they are less influenced by water temperature than other nitrogen fixers, UCYN-A may be less influenced by existing concentrations of bioavailable nitrogen.
Whatever the reason, this study makes it clear to scientists that the secrets of nitrogen fixation, and the limitations of UCYN-A, have yet to be fully illuminated.
[hr]
I’m a PhD candidate in Earth System Science at Stanford University, and I study how microbes in deep ocean sediments produce and consume greenhouse gases. I’m a native of the landlocked state of Minnesota, so I’ve always been fascinated by the ocean. When I’m not in the lab, I love to race triathlons, forward “The Onion” articles to friends and family, and hike with my hound dog Banjo.